- Share
- Share on Facebook
- Share on X
- Share on LinkedIn
These techniques also offer certain undeniable advantages (very high sensitivity, non-destructive measurement, ability to discriminate between isotopologues of the same total mass, to identify possible contaminants...). As an example, the measurement of Δ17O in CO2 by mass spectrometry requires distinguishing between two isotopologues with very close masses ("isobars"): 16O13C17O and 16O12C17O (hereafter noted 636 and 627 respectively). Current approaches to mass spectroscopy rely either on the chemical conversion of CO2 to O2 or on the detection of molecular fragments (oxygen atoms torn from the CO2 molecule in the mass spectrometer ionization source). The first of these approaches is far from being trivial, with more or less heavy experimental protocols conditioned to important investments. The second approach is based on the use of ultra-high resolution spectrometers costing several million euros, with very long term analyses (accuracy of 14 ppm after 20 hours of measurement).
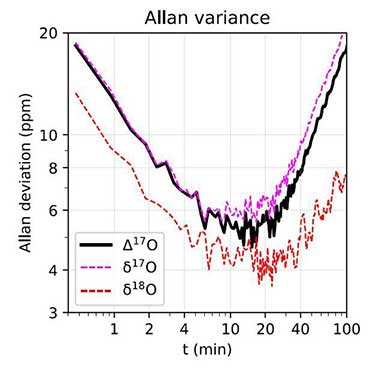
In theory, absorption spectroscopy can directly measure Δ17O by determining the abundance of the isotopologues 626, 627, and 628 without interference from 636, provided the necessary levels of precision (a few ppm) are achieved. That said, most commercial laser spectrometers today are still notable for their apparent simplicity of use and their ability to make rapid, real-time, and/or out-of-lab measurements. Undoubtedly, there is still progress to be made before absorption spectroscopy can claim to dethrone traditional isotopic techniques in terms of analytical precision.
The main problems to be solved concern the stability/reproducibility of short and long term measurements and the corrections of various "non-linearities" (pressure effects in particular). Despite this, the current consensus in the geochemical community is that optical techniques are on the verge of replacing mass spectrometry for demanding applications such as the measurement of isotopic anomalies, and that they constitute a highly strategic area of research in the coming years.
Actors
Samir KASSI
Erik KERSTEL
Daniele ROMANINI
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn