- Share
- Share on Facebook
- Share on X
- Share on LinkedIn
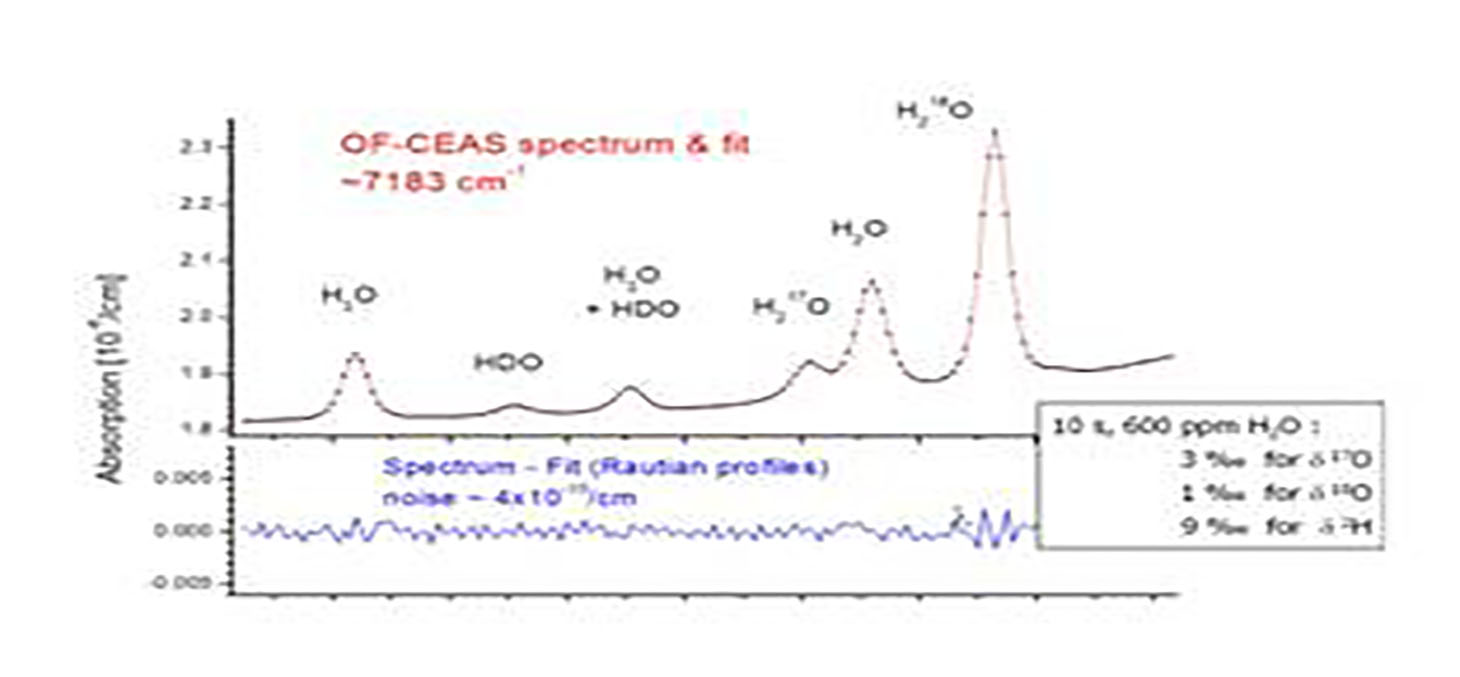
While the approximate abundances of these isotopes have been determined during nucleosynthetic processes in our solar system and on Earth, small variations in abundances occur during physical and chemical processes (due to small differences in mobility and binding energy).
The classic tool for measuring isotope abundance ratios is the isotope ratio mass spectrometer (IRMS), a magnetic sector mass spectrometer designed to measure the relative currents of ions over only a few mass channels, but with extreme accuracy. Although IRMS has come a long way since its inception by Nier and others in the late 1940s and now benefits from more than 50 years of commercial development, major drawbacks remain, including the size, cost, and complexity of the instrumentation and its incompatibility with direct measurement on a sticky molecule such as water. Our group is at the forefront of the development of laser-based spectroscopic techniques for the measurement of isotope ratios.
The actors
Samir KASSI
Erik KERSTEL
Daniele ROMANINI
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn