- Share
- Share on Facebook
- Share on X
- Share on LinkedIn
Thus, for example, the observation that atmospheric CO2 has seen its δ13C value decrease over the past 150 years as its concentration increases allows us to attribute most of this increase to fossil sources, which are significantly lower in 13C than other carbon sources.
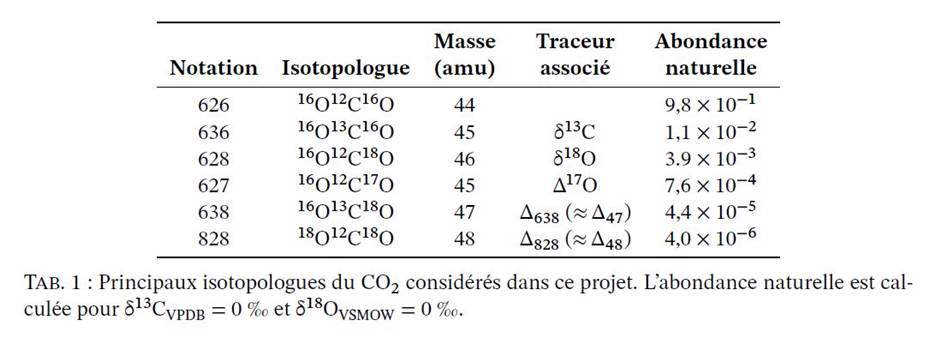
However, natural systems are complex and "classical" isotopic tracers (δ13C, δ18O, δ2H,...) are often insufficient to account for all the parameters we wish to study. For example, the oxygen-18 composition of marine carbonates from sedimentary cores from all the world's seas clearly varies according to quasi-periodic cycles of 100,000 and 40,000 years over the last 2.6 million years. It has long been known that these cycles reflect both changes in ocean temperature and changes in ocean isotopic composition (δ18O, which varies with ice sheet volume), but it is often difficult to quantify precisely the respective shares of these two factors. In addition to the classical isotopic tracers, the development of new tracers has progressively enriched the range of tools to study these complex systems. For example, the oxygen-17 anomaly, noted Δ17O, is defined as the deviation of the 17O/16O ratio measured in a sample from phenomenological laws that describe the distribution of the three stable oxygen isotopes (16O, 17O, 18O) in different molecules (O2, H2O, CO2, C32O-, S42O-,...).
Accurately measuring Δ17O provides additional information to complement other isotopic observations (e.g., δ18O). Isotopic clumping* refers to another class of isotopic tracers, defined as statistical anomalies with respect to a purely random ("stochastic") distribution of isotopes in a given sample. The best known of these tracers is isotopic "clumping" in CO2 and carbonate minerals ("Δ17"), defined as the statistical anomaly associated with CO2 isotopologues of mass 47, primarily consisting of 16O13C18O. In many natural carbonates, the value of Δ47 is directly related to its crystallization temperature.
To return to the previous example, if we are able to measure precisely, in a marine carbonate, both δ18O and Δ47, it becomes possible to quantitatively distiguish between the effects of temperature and those of the isotopic composition of water. These new tracers have grown rapidly over the last twenty years, and their applications cover a very wide range of scientific fields (paleo-climatology, diagenetic processes, petrology, atmospheric chemistry, methanogenesis, biocalcification, paleohydrology, cosmochemistry...). Their measurement is usually based on advanced mass spectrometry techniques associated with specialized analytical know-how (sample preparation protocols, standardization of measurements, etc.). The Ile-de-France region has internationally recognized expertise in the measurement of isotopic clumping, with the only two French laboratories that currently have infrastructures for Δ47 analyses (Laboratoire des Sciences du Climat et de l'Environnement; Institut de Physique du Globe de Paris).
Actors
Samir KASSI
Erik KERSTEL
Daniele ROMANINI
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn