- Share
- Share on Facebook
- Share on X
- Share on LinkedIn
Biobased thin films with antimicrobial activity
Samantha MICCIULLA, in collab. with Leonardo CHIAPPISI (Institut Laue-Langevin, Grenoble)
The self-assembly of polymeric thin films is largely exploited to enrich various substrates with tailored functionalities. The concept of surface modification by either chemical grafting or physical adsorption of macromolecules is rather simple and highly versatile, it is suitable for a large selection of species under various thermodynamic conditions, resulting in an equally wide range of internal structure, morphology and mechanical properties of the final films, while preserving the bulk substrate properties. Besides structure and mechanics, the biological functions of a surface can be tuned, including the introduction of antimicrobial properties, which are of highly relevance to prevent nosocomial infections in hospitals, but also in our daily life.
The research project aims at using biobased and biodegradable polymers to create antimicrobial coatings. We tackle the challenge of obtaining stable antimicrobial films under physiological conditions, including low pH and complex media, but also understanding the correlation between physico-chemical film properties and their interaction with the outer surface of bacterial cells. Given its abundance as a waste food product, its good filmability and biodegradability, we will make use of chitosan as simple (monolayers) up to complex multilayered films in combination with other negatively charged compounds, and verify the correlation between film properties and biological functions against bacterial cell proliferation.
Chitosan-based smart hybrid materials: a physico-chemical perspective
Giuseppe Cavallaro, Samantha Micciulla, Leonardo Chiappisi and Giuseppe Lazzara
Journal of Materials Chemistry B, 2021, 3, pp.594-611 ⟨10.1039/D0TB01865A⟩
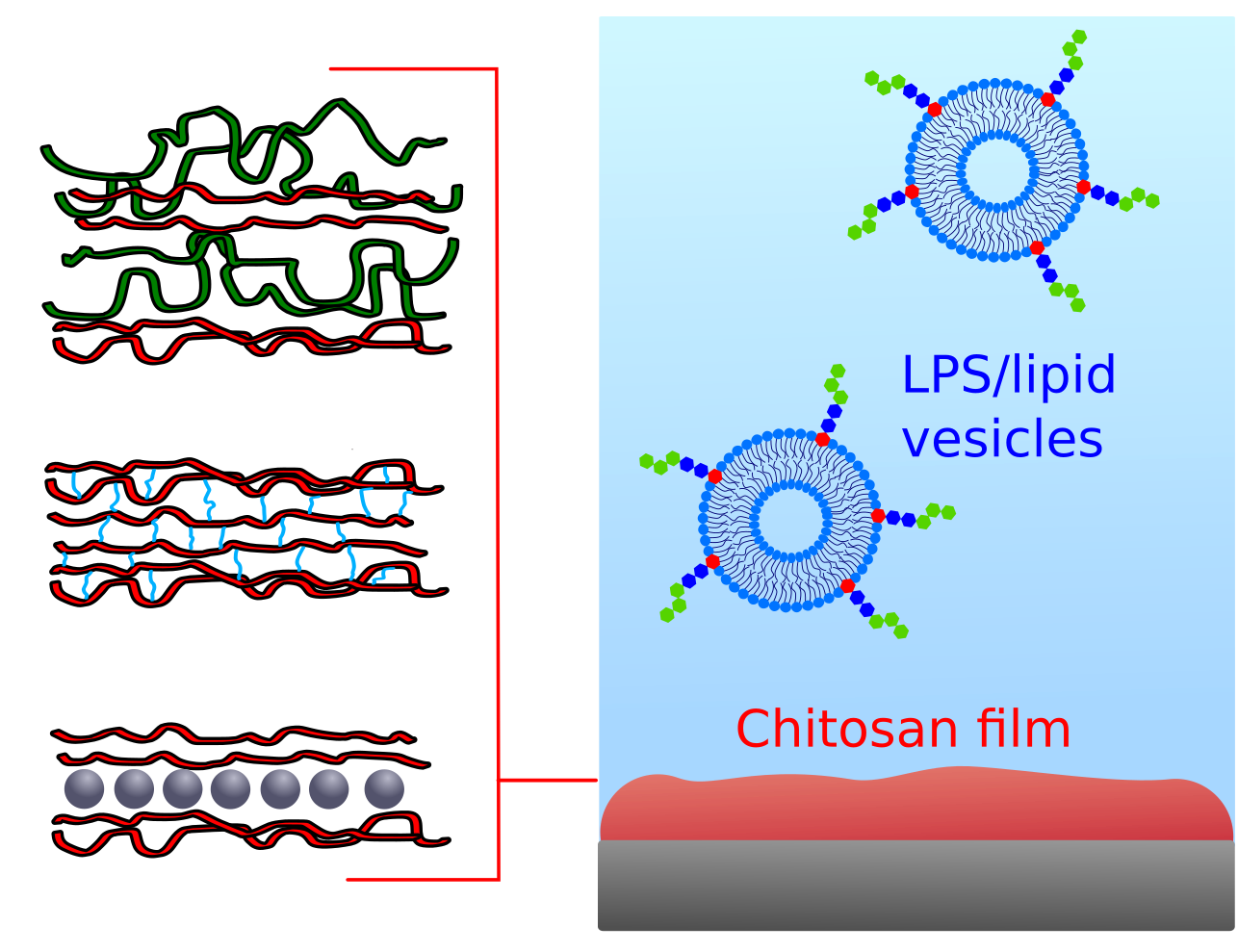
Structure-function relation in biomimetic membranes. Focus on Gram-Negative bacteria
Samantha MICCIULLA, in collab. with Nicolo PARACINI (Institut Laue-Langevin, Grenoble), Emanuel SCHNECK (Technische Universität Darmstadt, Germany), Cedric LAGURI (Institut de Biologie Structurale, Grenoble)
Antimicrobial resistance consists of a series of adaptation mechanisms which bacteria actuate to survive threatening conditions, leading to structural and functional rearrangements. The widespread dispensing, misuse and outright abuse of antibiotics is responsible for the development of many resistant strains, many of which being Gram-negative bacteria. Beside the alteration of the complex biological machinery which modify protein expression, lipid production and enzymatic activity, such bacteria protect themselves by a highly impermeable double-envelope membrane, acting as molecular sieves against most antibiotics available today. The design of new drugs which are able to overcome such barrier to reach the nucleus and kill bacteria needs a deep knowledge of the structure of such interfaces and how their stability can be compromised.
This research project focuses on the physico-chemical investigation of Gram-negative bacterial membranes at different levels of complexity, from single lipid layers up to double bilayers with embedded transmembrane proteins. The systems will be reconstituted at liquid and solid substrates to explore their properties under physiological conditions and upon interaction with model drugs (single molecules or drug carriers). In order to reproduce membrane-membrane interactions and biofilm formation, extensive work will be dedicated to the design of new experimental approaches to control their mutual separation distance on a nanometer scale, screening a broad range of interactions forces.
Lipopolysaccharides at Solid and Liquid Interfaces: Models for Biophysical Studies of the Gram-negative Bacterial Outer Membrane
Nicoló Paracini, Emanuel Schneck, Anne Imberty, Samantha Micciulla
Advances in Colloid and Interface Science, 2022, 301, pp.102603. ⟨10.1016/j.cis.2022.102603⟩
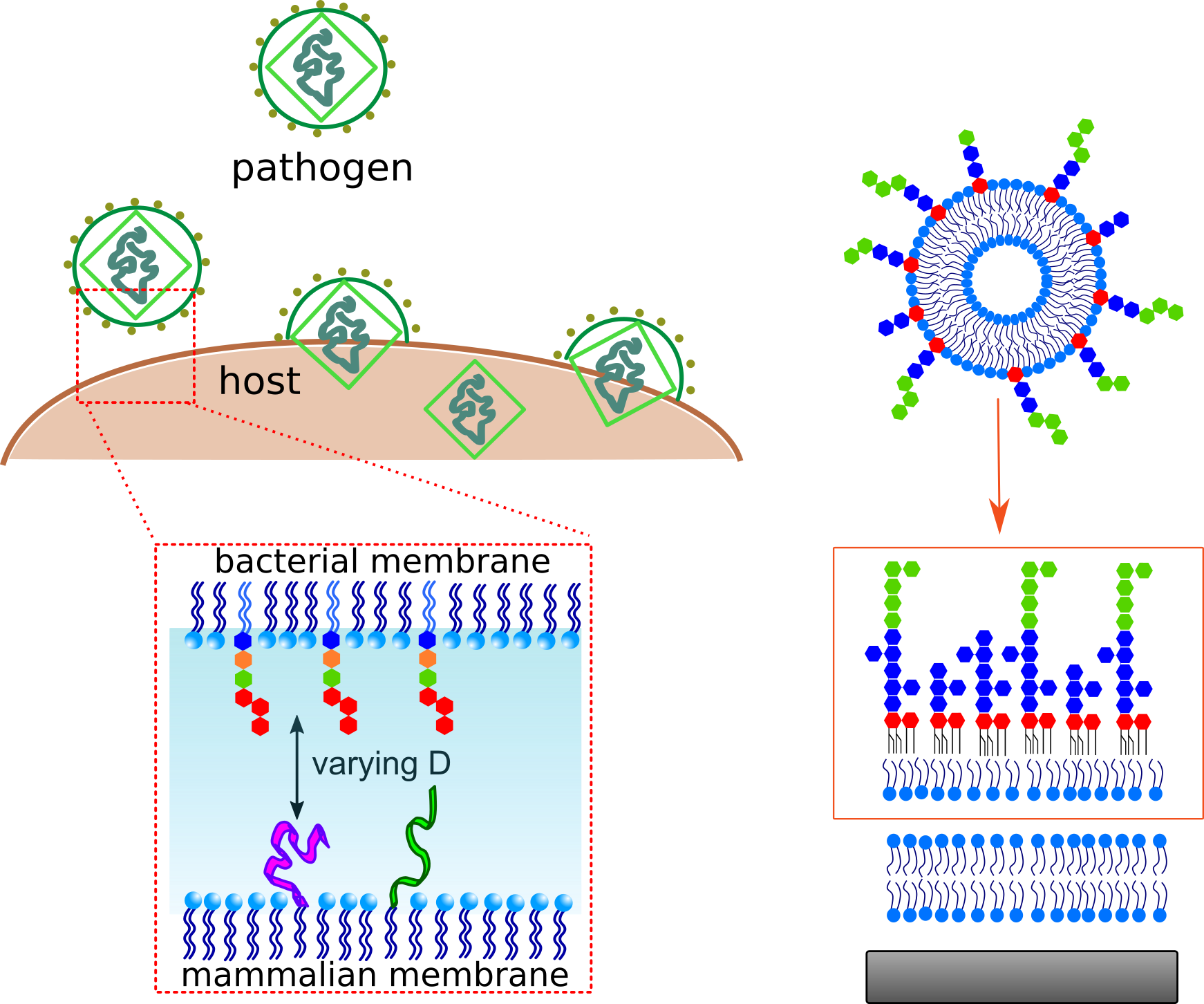
Interactions between polymer brushes and biological objects
Lionel BUREAU, in collab. with Delphine DEBARRE and Daria TSVIRKUN (MC2 team), Chaouqi MISBAH (EcCEL team), Martial BALLAND and Giovanni CAPPELLO (MicroTiss team)
This topic relies on the design and use of polymer brushes (layers made of macromolecules bound by one end to a surface) to tune the interactions between the surface of a solid and biological cells in its vicinity. We focus on two different situations of interest.
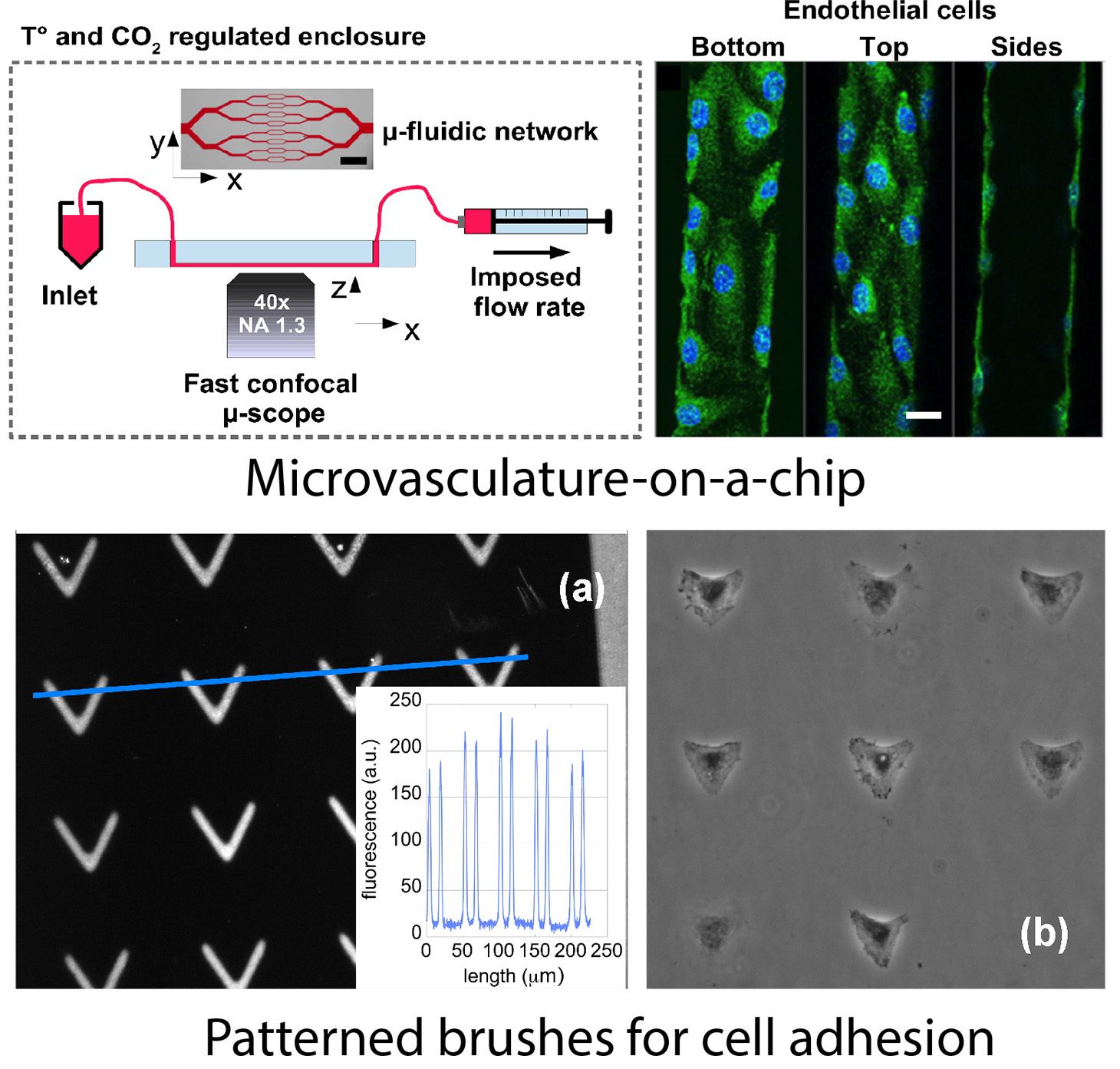
(i) The first one is related to the rheology of blood in the microvascular system: we study the role played by the endothelial glycocalyx, a micron-thick brush-like polymer structure that lines the lumen of blood vessels in vivo, and probe its effect on the dynamics of flowing blood cells and on the response of endothelial cells to mechanical stimuli. This is done by combining microfluidics and in situ cell culture, in order to design microvasculature-on-a-chip.
Funding: CNES - Programme “Dysfunction endothéliale”
Microvasculature on a chip: study of the Endothelial Surface Layer and the flow structure of Red Blood Cells
Daria Tsvirkun, Alexei Grichine, Alain Duperray, Chaouqi Misbah, Lionel Bureau
Scientific Reports, 2017, 7, pp.45036. ⟨10.1038/srep45036⟩
(ii) Our second field of application of polymer brushes is the control of adhesion of mammalian cells on a surface. We have developed a technique allowing to design brushes of poly(N-isopropylacrylamide) (PNIPAM, a thermoresponsive polymer) that exhibit adhesive patterns of well controlled geometry. We have shown that such patterned brushes can be efficiently used to create regular arrays of cells following a given geometric constrain.
Funding: ANR SPOC
Thermoresponsive micropatterned substrates for single cell studies
Kalpana Mandal, Martial Balland, Lionel Bureau
PLoS ONE, 2012, pp.e37548. ⟨10.1371/journal.pone.0037548⟩
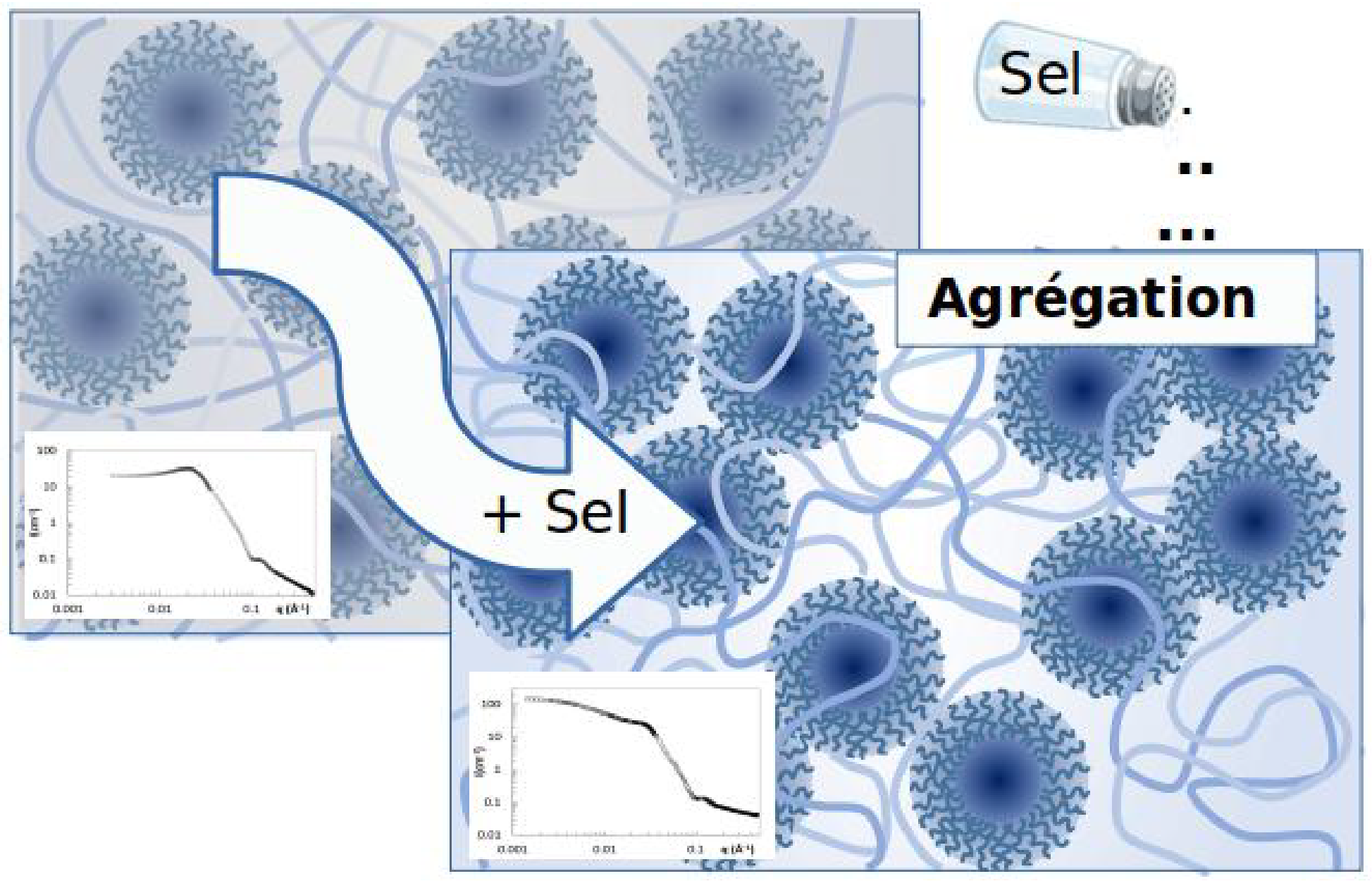
Assemblies of hyaluronan with colloids and proteins
Isabelle MORFIN, Marie PLAZANET and Judith PETERS, in collab. with Isabelle GRILLO and Sylvain PREVOST (Institut Laue-Langevin, Grenoble) and Jérôme COMBET (Institut Charles Sadron, Strasbourg)
Using X-ray and neutron scattering techniques, we study multi-component structures of hyaluronan associated with proteins, charged or neutral colloids. We observe upstream of any application or for specific applications the role of intrinsic parameters of hyaluronan, such as the mass or stiffness of the chains, the role of water molecules and associated hydrogen bonds, as well as the balance between electrostatic, hydrophobic or depletion forces.
Chain conformation: A key parameter driving clustering or dispersion in polyelectrolyte – Colloid systems
I. Grillo, I. Morfin, J. Combet
Journal of Colloid and Interface Science, 2020, 561, pp.426-438. ⟨10.1016/j.jcis.2019.11.010⟩
Bubble growth in complex heated media
Élise LORENCEAU and Irène WANG, in collab. with Carlos ARAUZ MORENO and Keyvan PIROIRD (Saint-Gobain Research)
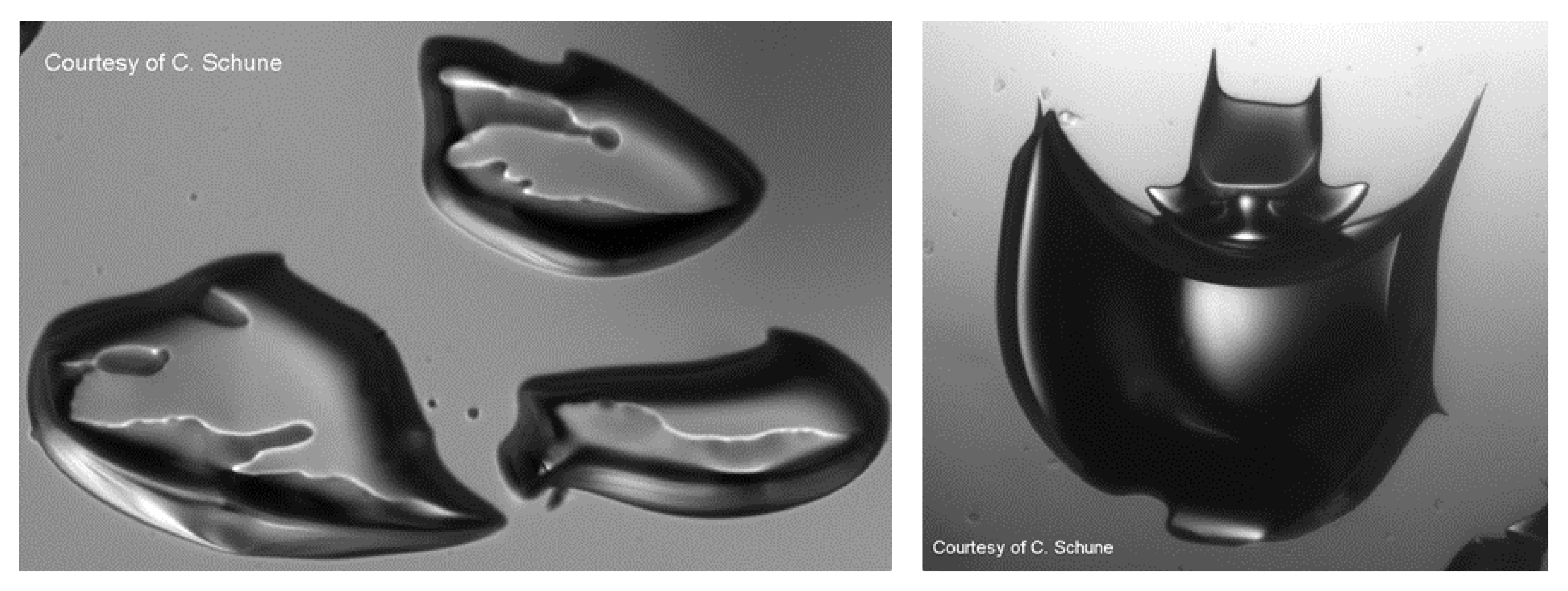
It is common to observe bubbles stuck in complex materials (such as transparent hydroalcoholic gel or laminated glass in windshields). These bubbles, which degrade the optical quality of the material, can appear during normal use of the material or during a quality test at high temperature. The kinetics of bubble growth in a liquid or complex medium supersaturated with gas and suddenly brought into contact with atmospheric pressure -such as champagne- is well known. Yet, there are far fewer studies on the effects of an increase in temperature on the growth of a bubble in a complex medium. The subject is however rich since a multitude of parameters (diffusion coefficient, rheology, solubility) depend on the temperature. To tackle this issue, we built two transparent autoclaves allowing us to subject samples to pressure and temperature cycles and to follow the growth of gas inclusion. The stress distribution around the bubbles can be followed by birefringence. The images below illustrate centimetric bubbles with strange shapes that appeared during the heating of a polymer confined between two glass plates.
Funding: Saint-Gobain Research
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn