- Share
- Share on Facebook
- Share on X
- Share on LinkedIn
Microscopy at traction forces
Objective :
Measure the forces exerted by cells on their environment
Implementation :
The cell is deposited on a deformable hydrogel, doped with fluorescent tracers, whose displacements are followed by fluorescence microscopy (Figure 3). The analysis of these displacements gives access to the local deformations of the environment, and indirectly, to the forces exerted by the cells.
Reference :
Lafaurie-Janvore, J. et al. ESCRT-III Assembly and Cytokinetic Abscission Are Induced by Tension Release in the Intercellular Bridge. Science (80-. ). 339, 1625–1629 (2013).
Soft Patterning
Objective :
Simultaneously control the geometry and mechanical properties of the cell environment to manipulate cell adhesion, spreading and architecture
Implementation :
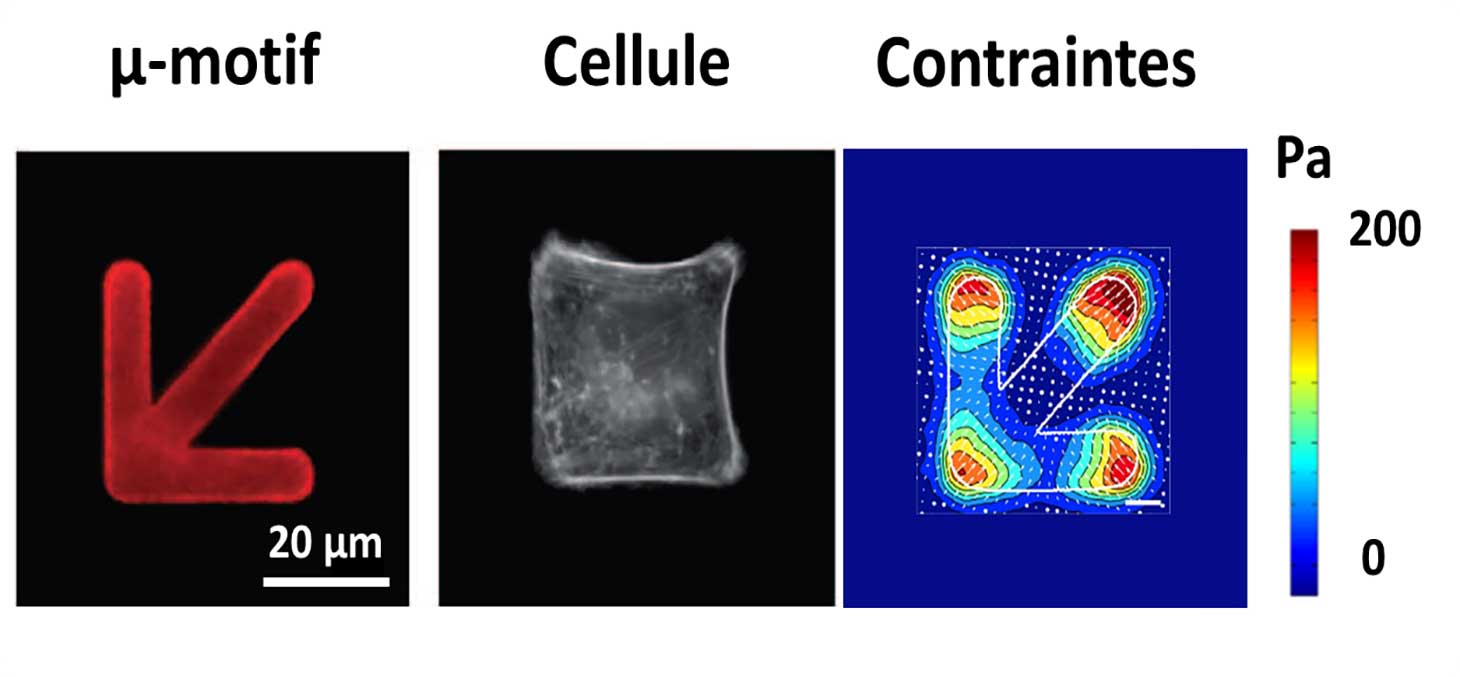
A biochemical pattern is printed by photolithography on the deformable hydrogel, in order to alternate adhesive and repulsive areas for the cells. The size and geometry of the pattern is chosen by the experimenter and the pattern is replicated a large number of times to multiplex the experiment on many cells.
Types of Observation :
The cells adapt their geometry to the imposed pattern, thus inducing a structural and functional reorganization. This reorganization is measurable by optical video-microscopy and tensile force microscopy (Figure 3). Several interacting cells can be placed on the same pattern to study the propagation of biological and mechanical signals between neighboring cells.
Reference :
Tseng, Q. et al. A new micropatterning method of soft substrates reveals that different tumorigenic signals can promote or reduce cell contraction levels. Lab Chip 11, 2231–2240 (2011).
Microfabrics
Objective :
Create self-organizing and functional three-dimensional tissues.
Implementation:
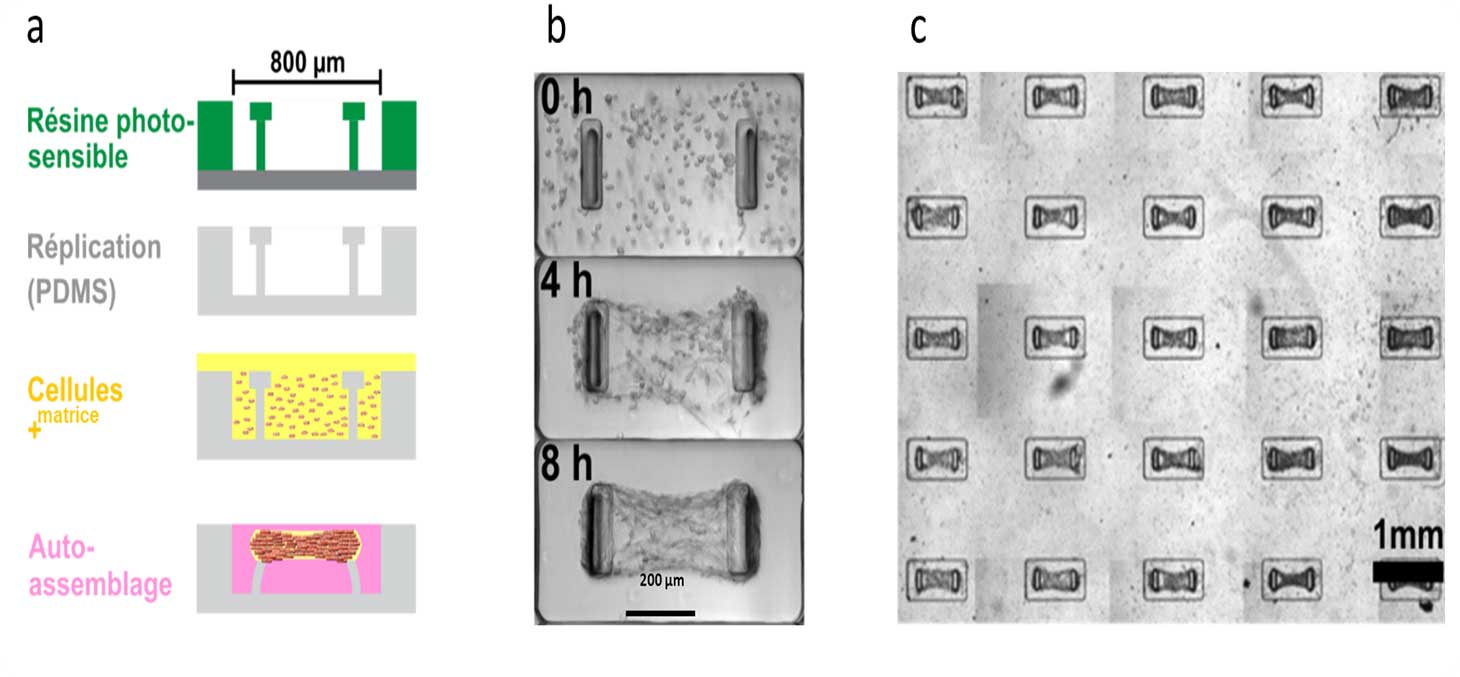
MicroTiss develops techniques to generate free (spheroid) or constrained (microtissue) 3D tissues. The first method consists in culturing cells on a repulsive substrate, which promotes the spontaneous formation of a spherical multicellular aggregate. The second method consists of depositing a mixture of cells and extracellular matrix in a micro-culture chamber in the presence of deformable pillars (Figure 4). In this case, the cells self-organize to form a 3D micro-tissue, suspended from the pillars.
Types of Observation :
The formation of the micro-tissues can be observed in real time by video-microscopy, under different experimental conditions (type, number and age of the cells, type and density of the extracellular matrix, ...). The pillars being deformable and of known rigidity, their deflection indicates in real time the forces generated by the microtissue.
Reference :
Boudou, T. et al. A Microfabricated Platform to Measure and Manipulate the Mechanics of Engineered Cardiac Microtissues. Tissue Eng. Part A 18, 910–919 (2012).
Ramade, A., Legant, W. R., Picart, C., Chen, C. S. & Boudou, T. Microfabrication of a Platform to Measure and Manipulate the Mechanics of Engineered Microtissues. in Methods in Cell Biology (ed. Théry, M. P. and M.) 121, 191–211 (Elsevier Inc., 2014).
Micro pressure sensors
Objective :
Measure the mechanical stress inside a three-dimensional fabric.
Implementation :
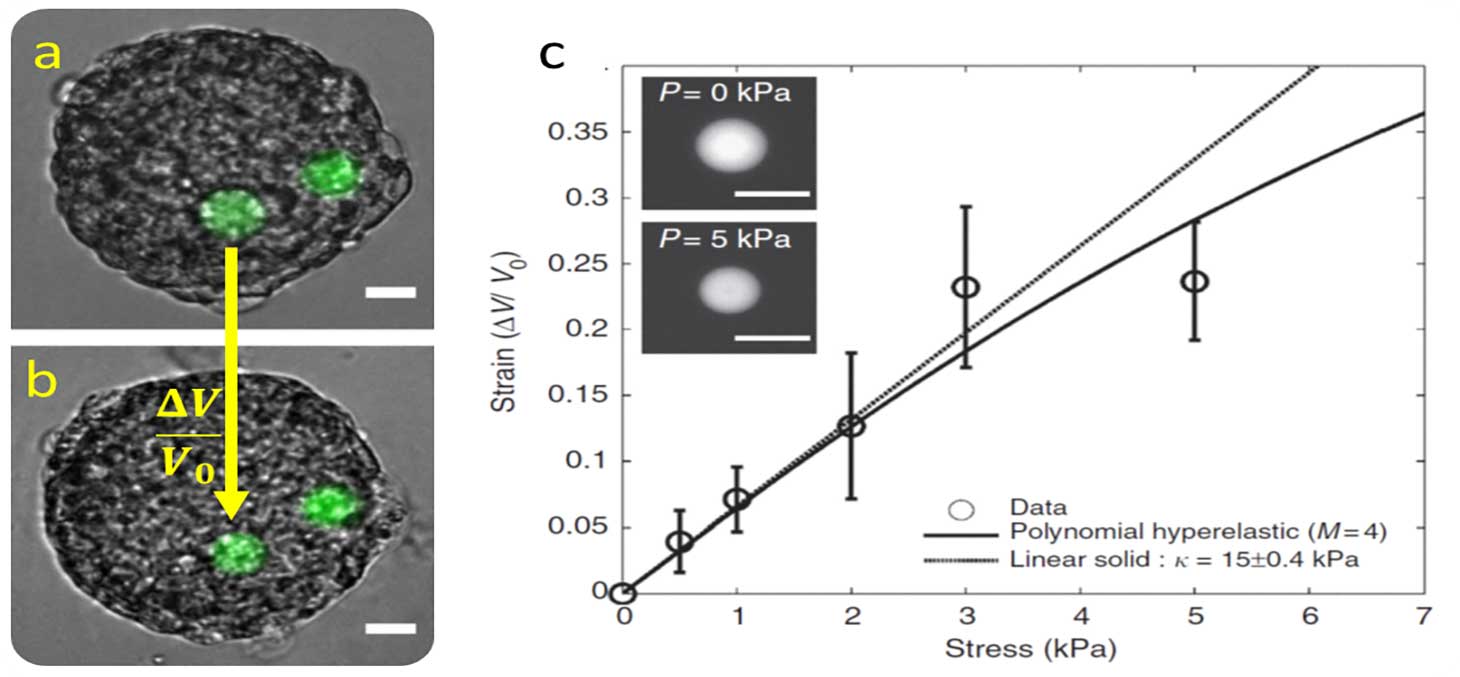
We realize micro pressure sensors that mimic the size, stiffness and surface state of a cell. Thanks to these characteristics, the micro-sensor is spontaneously incorporated by biological tissues.
Stress measurement :
The micro-sensors are polyacrylamide beads, deformable by fluorescence. From the observations by fluorescence microscopy, the deformation caused by the tissue is quantified. A universal calibration curve allows to link this deformation to the stress that the tissue exerts on the bead (Figure 5).
Reference :
Dolega et al.; “Cell-like pressure sensors reveal increase of mechanical stress towards the core of multicellular spheroids under compression”; Nature Comm. (2017)
Optogenetic control of contractility
Objective :
Modulate cell contractility in time and space.
Implementation :
The use of drugs - such as blebbistatin - can affect cell contractility. The use of genetically modified cells adds the possibility to modulate their contractility locally in space and time.
Control of contractility by light:
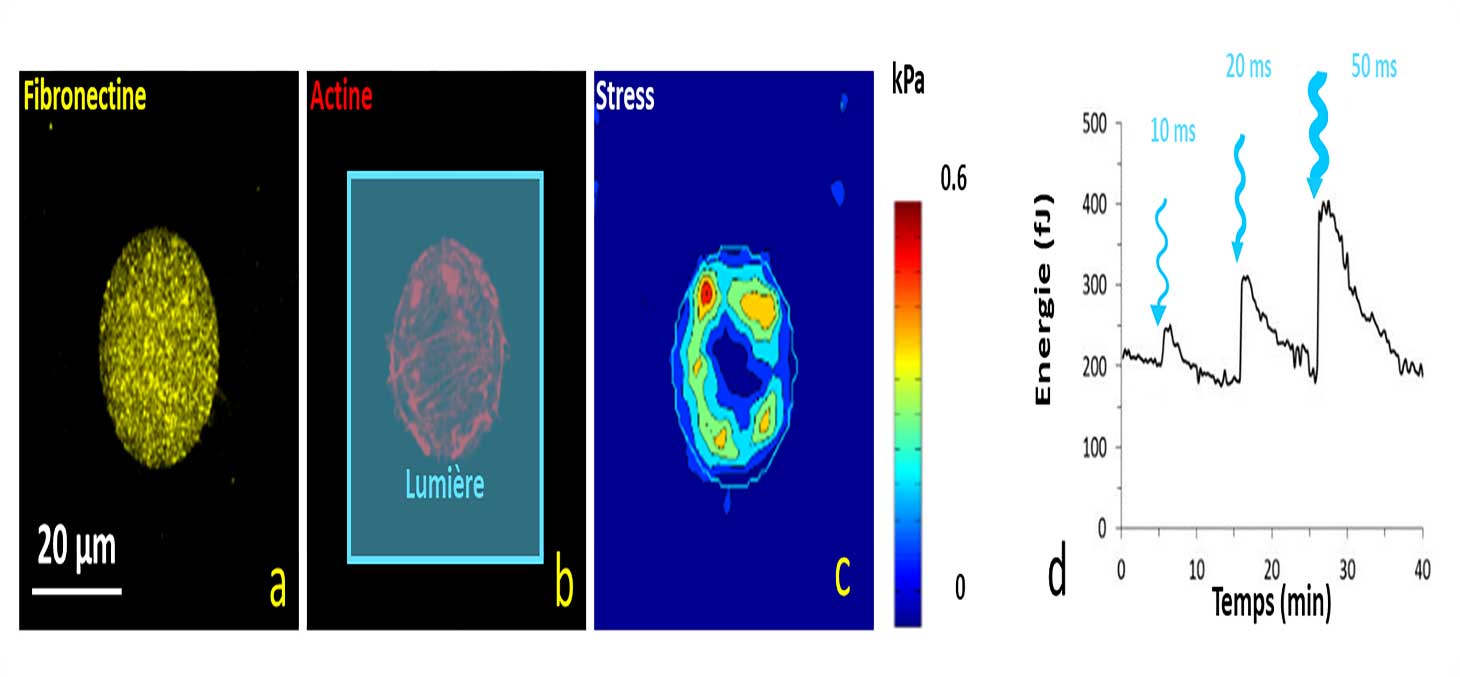
When optogenetically modified cells are illuminated with blue light, their contractility increases significantly. Figure 6 shows an example of a cell that contracts following periods of illumination. The magnitude of the effect is proportional to the energy of the light pulse.
Reference :
- Andersen, T. et al. Cell size and actin architecture determine force generation in optogenetically activated adherent cells. bioRxiv (2022).
- Méry, A. et al. Light-driven biological actuators to probe the rheology of 3D microtissues. BioRxiv (2022). doi:10.1101/2022.01.05.475039
- Hennig, K. et al. Stick-slip dynamics of cell adhesion triggers spontaneous symmetry breaking and directional migration of mesenchymal cells on one-dimensional lines. Sci. Adv. 6, eaau5670 (2020).
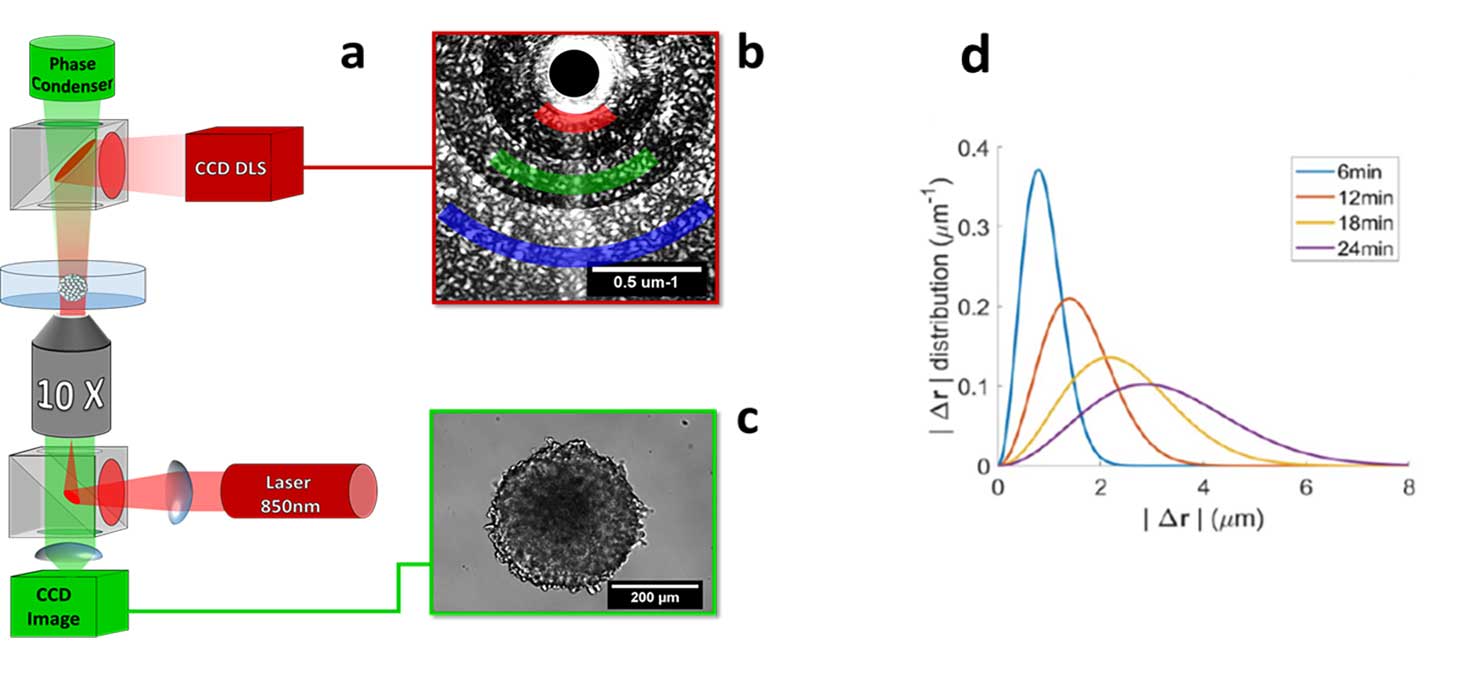
Dynamic light scattering
Objective :
To quantify the cellular dynamics in an opaque three-dimensional tissue
Problem :
Cells are scattering objects. This makes thick tissues opaque, where it is difficult to make observations beyond the first cell layers. However, the scattered photons having interacted with the cells still carry information that can be exploited.
Light scattering and dynamic interference :
When illuminated by a coherent source, 3D tissues produce an interference pattern, which varies over time. By analyzing the dynamics of this interference pattern, it is possible to reconstruct the spatial distribution and velocity field of cells within the tissue.
Reference :
B Brunel et al., “Structure and dynamics of multicellular assemblies measured by coherent light scattering”, New Journal of Physics (2017)
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn