- Share
- Share on Facebook
- Share on X
- Share on LinkedIn
Thesis defence
On December 11, 2023
Agathe Nidriche
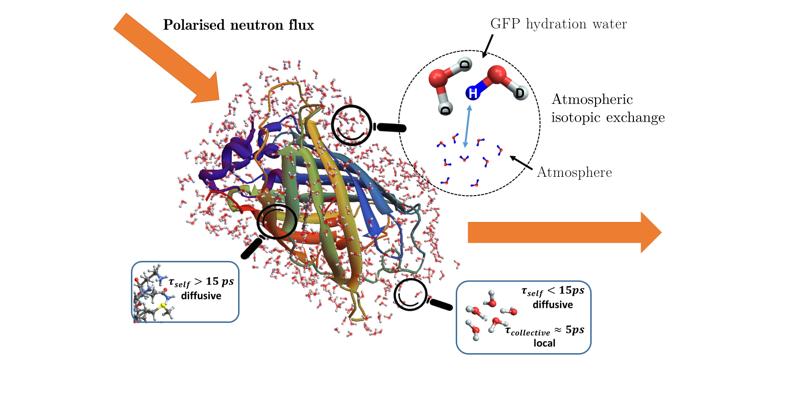
Proteins undergo dynamics that span several orders of magnitude in time, englobing the femtosecond to microsecond timescale which are investigated with neutron scattering techniques. It is a valuable tool to study the dynamics of hydrogen nuclei in proteins. However, neutron scattering is not just a probe for self-diffusion, but also encodes collective dynamics through its division into an incoherent (self) and coherent (self and collective) contributions. It is always stated that coherent scattering is negligible in hydrogen-rich systems like proteins. In order to challenge this assumption, the advent of neutron polarisation set-ups for the Time-Of-Flight technique permits us to investigate separately both contributions in powder-state Green Fluorescent Protein (pGFP) and its per-deuterated counterpart (dGFP) hydrated in.
First and foremost, polarized neutron diffraction drives us to understand the extent of D/H exchange between the atmosphere and the hydration shell of the protein. This is then pushed forward with a pico-second dynamical study with polarised quasi-elastic neutron scattering, where we prove that coherent scattering is non-negligible due to D-bond collective relaxation in the hydration water of both proteins. The features of this collective relaxation share similarity with recent studies on liquids and polymers. In a more general manner, we have brought the use of a theoretical framework for complex systems with self-similar dynamics, as well as new experimental techniques, to enhance studies of biomolecules with QENS and question usual assumptions.
Jury :
- ARBE Arantxa (Rapporteur)
- STADLER Andreas (Rapporteur)
- GEISELMANN Hans (Examinateur)
- NILSEN Goran (Examinateur)
- SMITH Jeremy C (Examinateur)
- PETERS Judith (Directrice de thèse)
Date
14:00
Localisation
LIPhy, salle de conférence
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn