- Imprimer
- Partager
- Partager sur Facebook
- Partager sur X
- Partager sur LinkedIn
Séminaire
Le 5 mars 2026
Marco Canepari (Imov)
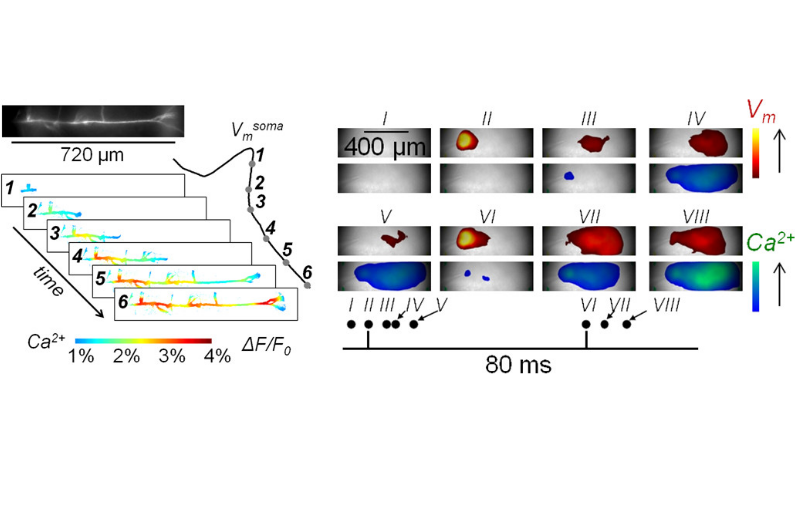
The brain slice is a living tissue, kept under physiological conditions for up to 6 hours, where neurons and local circuitries are preserved as in vivo. In this preparation, it possible to inject Ca2+ indicators and voltage sensitive dyes (VSDs) into individual neurons and perform ultrafast (5-40 kHz) imaging to record membrane potential (Vm) and Ca2+ transients, associated with stimulated activity, in specific sub-cellular compartments such as axons, dendrites and synaptic spines. Ca2+ indicators and VSDs can be also loaded in the whole slice, losing the ability to resolve single cells but gaining the possibility to monitor network activity. Vm imaging allows recording action potentials and synaptic potentials while Ca2+ influx, triggered by Vm signals, can be imaged and correlated with electrical activity. In fundamental neuroscience, this approach permits the unravelling of complex ion channels synergies as well as the interactions with other molecules underlying neuronal function. In preclinical research, the same strategy allows untangling dysfunctions caused by genetic mutations in animal models of human disease. Finally, combining Vm and Ca2+ imaging is a powerful tool to investigate the effect of drugs on neuronal excitability, synaptic transmission and global network activity. In the project SynBin, we will use our high-resolution imaging approaches to explore diversity of synaptic spines in the same cerebellar Purkinje neuron. This project challenges classical learning theories assuming that synapses of a given type are identical except for variations of receptor numbers resulting from plasticity, whereas evidence suggests that synapses have specific molecular identities, implying novel hypotheses regarding the mechanisms of synaptic computations, plasticity and their role in learning and memory. In the project Chlorzowill, we will study an animal model of the Williams-Beuren syndrome, a rare genetic disease caused by deletion of several contiguous genes resulting in neurological and cardiovascular alterations. Grounded on the evidence that the disease is associated with the down-regulation of BK K+ channels, we will explore neuronal excitability on the animal model and the potential therapeutic effect of chlorzoxazone targeting this type of channels. Finally, whole-slice combined Vm and Ca2+ imaging can be exploited to rapidly and efficiently assess the acute effects of drugs in the long industrial lead-selection process that precedes clinical trials. A technology transfer project is ongoing on this topic.
Date
11:00
Localisation
LIPhy, salle de conférence
- Imprimer
- Partager
- Partager sur Facebook
- Partager sur X
- Partager sur LinkedIn