- Share
- Share on Facebook
- Share on X
- Share on LinkedIn
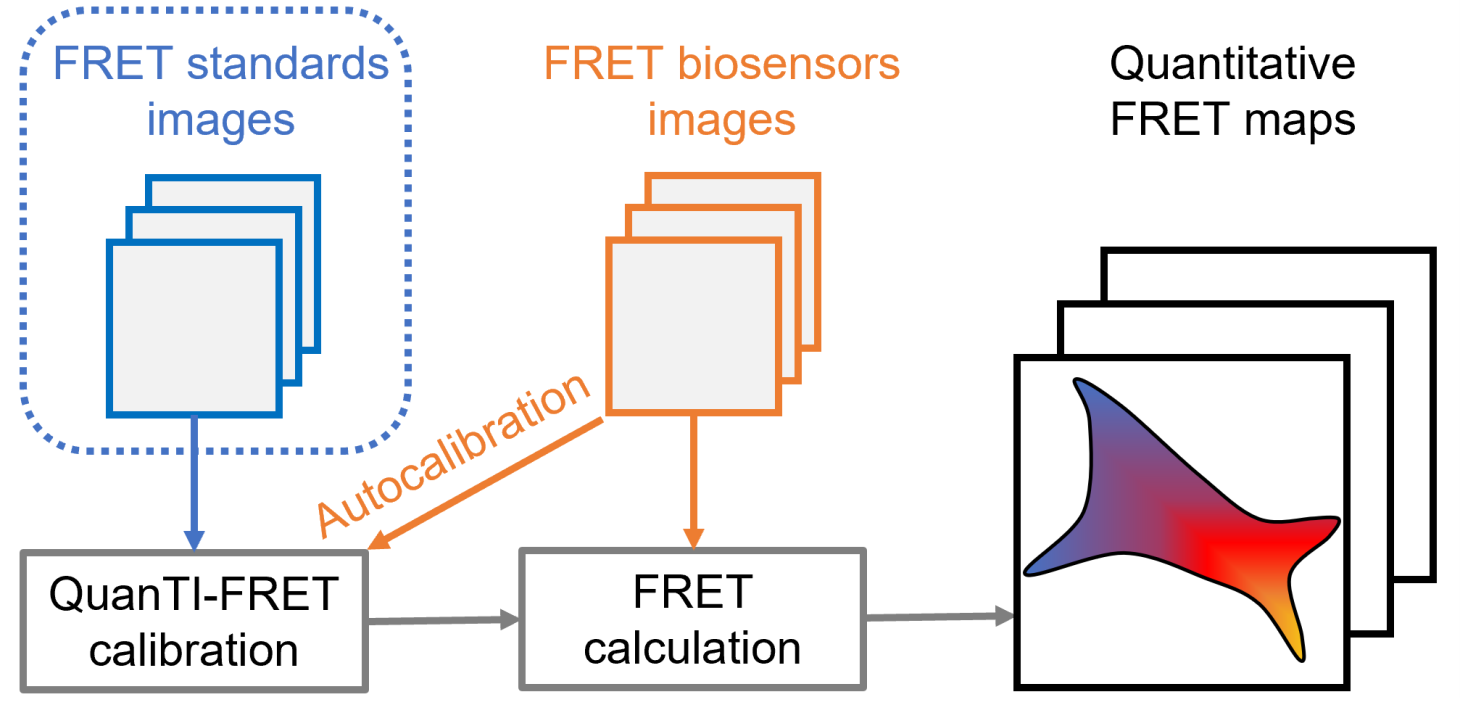
Being able to measure the biochemical activity of a target protein in a living cell in a spatially and temporally resolved manner is a challenge. This is not possible with classical molecular biology methods, however, new tools have recently emerged, namely fluorescent biosensors. Most of them are based on the principle of "Förster Resonance Energy Transfer" (FRET), i.e., the transfer of energy between two fluorophores allowing to probe distances of the order of a few nanometers and thus changes in protein conformation. These biosensors have enormous potential but their development is hampered on the one hand by the difficulty to develop them and on the other hand by the difficulty of measuring FRET reliably in living cells. To overcome this latter issue, we have developed, in collaboration with the team of Don Lamb (LMU Munich), a new calibration method that allows the measurement of absolute FRET values and thus opening the way to new experiments. In addition, this method gives access to the relative stoichiometry between donor and acceptor molecules and hence allows the quantitative measurement of inter-molecular FRET biosensors (where the stoichiometry is not fixed). This method accessible through an open Python code (https://gricad-gitlab.univ-grenoble-alpes.fr/liphy/quanti-fret) and a plugin for Napari free software (https://napari-hub.org/plugins/quanti-fret.html).
People involved
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn