- Share
- Share on Facebook
- Share on X
- Share on LinkedIn
Thesis defence
On December 20, 2023
Oksana Kirichuk
Thesis supervisors: Delphine DEBARRE, Lionel BUREAU, Ralf RICHTER (University of Leeds, UK)
Jury members: Olivier THEODOLY (Inserm), Marta BALLY (Umeå University, Sweden), Emmanuelle PLANUS (IAB, UGA), Liliane COCHE-GUERENTE (DCM, UGA)
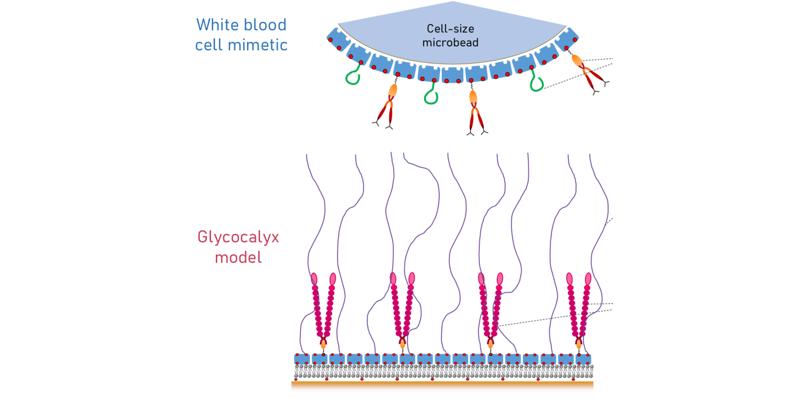
Abstract: Cell adhesion to the blood vessel wall is a complex, highly regulated physiological process. Red blood cells must repel from the blood vessel wall to prevent blood clotting while immunecells can be recruited from the vascular system to migrate into surrounding tissues. Cell adhesion hinges on the critical role played by the glycocalyx, a soft gel-like layer coating the vascular wall. However, how glycocalyx mechanical (softness, thickness) and biochemical (the composition and the density of surface receptors) properties affect this regulation is still poorly understood. Our hypothesis is that selective cell adhesion requires an intricate interplay of mechanical and biochemical cues. Elucidating the physical and molecular mechanisms that underpin selective adhesion directly in real blood vessels is challenging owing to the complexity and lack of control in in vivo systems. In my research, I aimed to construct an in vitro molecular interaction platform to facilitate mechanistic analyses. The platform combines a molecularly-defined model of the glycocalyx with mimetics of white blood cells under flow. While developing such a platform posed challenges, it offers the advantage of precise control over the physical and biochemical parameters of both the glycocalyx mimetic and cell mimetics. The newly developed glycocalyx model includes several key ingredients with tightly controlled properties: a brush of hyaluronan (HA, an essential component of the endothelial glycocalyx)is combined with P-selectin (an adhesion molecule on the endothelial cell surface critical for the homing of leukocytes). Building on previous experience in my research group, I employeda silica-supported lipid bilayer (SLB) bearing a monolayer of streptavidin (SAv), that can bind biotinylated molecules via biotin-SAv bonds. I introduce here a control of the in-plane mobility ofmolecules anchored to the fluid lipid bilayer using glutaraldehyde (GTA) as a cross-linking agent for SAv. Controlled grafting densities of one-end biotinylated HA chains of various lengths thencreate brushes of different mechanical properties. I also present a new methodology for quantitatively tuning the grafting density of smaller biotinylated molecules, which is deployed hereto control the grafting density of an ‘adapter protein’ for anchoring P-selectin. The new in vitro model of the glycocalyx thus affords control over the lateral mobility, the surface density and theorientation of two distinct functional molecules. The second key component of the newly developed platform consists of white blood cell mimetics, developed based on commercially available microbeads with the size of a cell and aSAv coating. I introduce a methodology for simultaneous grafting of two types of proteins onto the bead surface: biotinylated CD44 (a ligand expressed on leukocyte surfaces, interacting specifically with HA) and PSGL-1 (a ligand of P-selectin). Additionally, I present a method for controlling the surface density of each of these proteins. I use a combination of methods as monitoring and quality control tools of glycocalyx model formation and bead functionalization: quartz crystal microbalance with dissipation monitoring (QCM-D); spectroscopic ellipsometry (SE), reflection interference contrast microscopy (RICM); confocal microscopy with fluorescence recovery after photobleaching (FRAP) capabilities, and flow cytometry.This newly established platform provides a controlled environment for studying blood cell adhesion, effectively bridging the divide between cell-glycocalyx chemical interactions and themechanical aspects of cell migration under flow, including attachment and repulsion from the vascular wall. This platform holds the potential for expansion to encompass other surface adhesion molecules or to integrate multiple adhesion molecules, to gradually advance from the bottom up our understanding of the mechanisms governing cell adhesion to blood vessels.
Date
14:00
Localisation
LIPhy, salle de conférence
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn