- Share
- Share on Facebook
- Share on X
- Share on LinkedIn
Publication / Research
On February 28, 2023
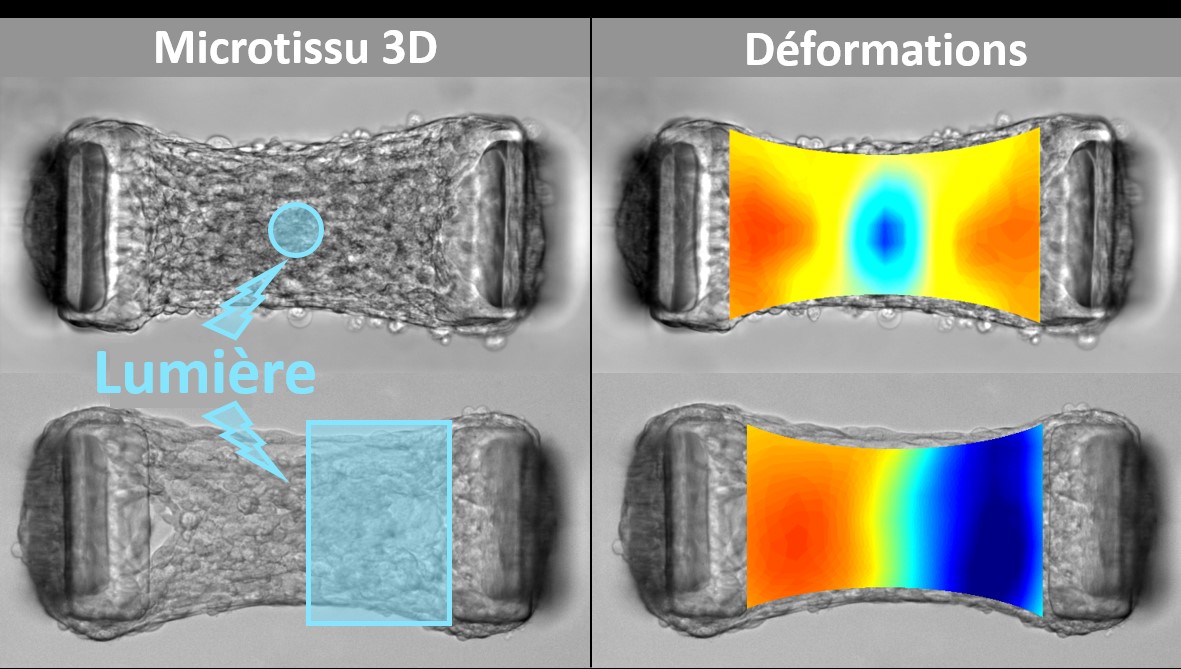
By combining tissue engineering and optogenetics, biophysicists have transformed cells into biological microactuators in order to study the spatio-temporal propagation of mechanical signals in biological tissues and to characterize the architecture and viscoelastic properties of these tissues.
In vivo, cells apply forces on their environment in order to move, divide or modify this environment, but also to communicate and coordinate with their neighbors at long distance. In standard approaches, the exploration of these mechanisms is done through external mechanical actuations that aim to mimic these mechanical signals. However, it remains difficult to assess how these external stimuli compare to the contractions that cells exert spontaneously. Biochemical treatments can modulate the cellular processes responsible for these signals, but their inability to target spatially defined areas of tissue and their low temporal resolution severely limit their potential. In this paper, a new approach is proposed to elucidate how cellular forces are generated, propagated and detected in physiological and pathological tissues.
Biophysicists from the Laboratoire Interdisciplinaire de Physique de Grenoble (CNRS - Univ. Grenoble Alpes) have sought to probe the mechanical properties of biological tissues "from the inside", as cells naturally do. To do this, they combined tissue engineering and optogenetics. Tissue engineering allowed them to generate three-dimensional microtissues, composed of fibroblasts encapsulated in collagen, suspended between two micropillars whose deflection allows to follow the tissue tension in real time. The optogenetic approach consists in genetically modifying fibroblasts in order to control the activity of a major regulator of their contractility by light. Thanks to the spatial and temporal resolution of the light stimulations, they induced local contractions in these microtissues, while measuring the resulting deformations. They thus quantified the viscoelastic properties of these microtissues, from the "point of view" of the cells, and demonstrated the potential of this approach to quantify the impact of collagen or fibrosis initiation on tissue elasticity. The ability to illuminate only a portion of the tissue allowed them to map local anisotropies in heterogeneous microtissues and influence the formation of these tissues. These results pave the way for spatio-temporal control of tissue formation while non-destructively mapping their rheology in real time, using their own constituent cells as internal actuators.
References:
Méry A, Ruppel A, Revilloud J, Balland M, Cappello G & Boudou T. Light-driven biological actuators to probe the rheology of 3D microtissues. Nat. Commun.14, 717 (2023). doi:10.1038/s41467-023-36371-w
Date
Contact
Thomas BOUDOU
Office 125
thomas.boudou univ-grenoble-alpes.fr (thomas[dot]boudou[at]univ-grenoble-alpes[dot]fr)
univ-grenoble-alpes.fr (thomas[dot]boudou[at]univ-grenoble-alpes[dot]fr)
More information
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn