- Share
- Share on Facebook
- Share on X
- Share on LinkedIn
Seminar
On January 20, 2025
Eloina Corradi (MICROTISS, LIPhy)
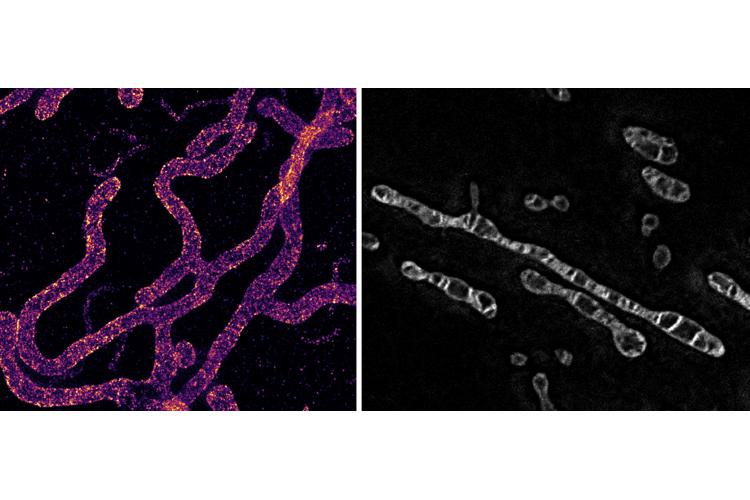
Mitochondria are double-membrane organelles that sustain the bioenergetic metabolism of cells by producing ATP at the level of the cristae, convoluted folds of the inner membrane. ATP production efficiency and mitochondrial morphology, including cristae architecture, are tightly linked. Consequently, by being coupled to mitochondrial function, the reshaping of mitochondria across spatial scales regulates the cellular bioenergetic and metabolic states. Mechanical stimuli have recently been shown to modulate cellular metabolism, thus impacting physiological (e.g. cell migration, proliferation, death) and pathological (e.g. cancer progression) processes.
By using a unique biomechanical tool to stretch cells compatible with super-resolution microscopy, we were able to show that mechanical stimulation drastically re-shape mitochondria across spatial scale. Indeed, a single mechanical stretch reshaped these organelles both at the macro and nanoscale, deforming and modulating both external and internal architecture. Overall, we show how mechanical forces reshape mitochondria at a spatiotemporal resolution never tackled before, further opening the horizon of mechano-metabolism based on cellular organelle re-shaping.
Date
11:00
Localisation
LIPhy, salle de conférence
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn