- Imprimer
- Partager
- Partager sur Facebook
- Partager sur X
- Partager sur LinkedIn
Séminaire
Le 7 février 2025
Thibault BRAM DIT SAINT AMAND (Microtiss, Liphy) // Antoine JALLON (MC2, Liphy)
Tuning the mechanics of fibrous tissues using optogenetics

Thibault BRAM DIT SAINT AMAND (PhD at Microtiss, Liphy)
Mechanical signals play a key role in the regulation of biological processes such as morphogenesis, homeostasis, or wound healing. At small scales, contracting cells can induce such signals, deforming and remodeling over long distances the extracellular matrix (ECM). allowing large-scale reorganization events such as tissue formation or repair. The understanding of the interactions between tissue contractility and its ECM can provide detrimental information on physiological mechanisms, but also pathologic mechanisms such as fibrosis. To investigate this feedback between cell contractility and ECM remodeling, one would need to simultaneously modulate and measure cell forces, while evaluating cytoskeletal and extracellular architecture.
To this end, we use NIH 3T3 cells expressing a Cry2-CIBN optogenetic probe to control cellular contractility. When placing a mixture of these cells and collagen in PDMS microwells containing flexible micropillars, we succeed in engineering microtissues, trigger their contraction and measure the forces generated through the deflection of the pillars (Fig. 1). Altogether, our system provides unique opportunities to elucidate how mechanical cues dynamically regulate tissue formation and function.
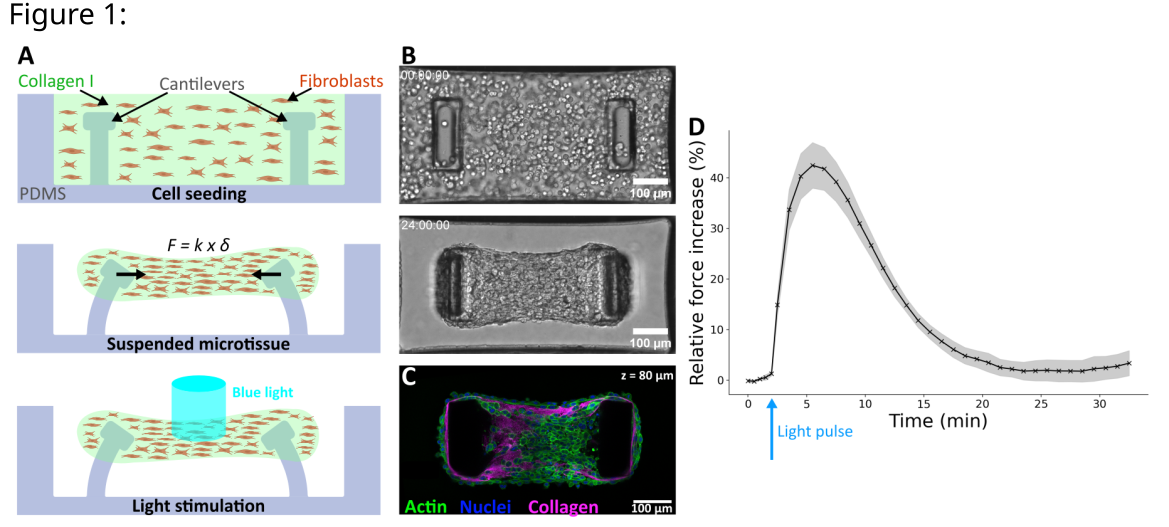
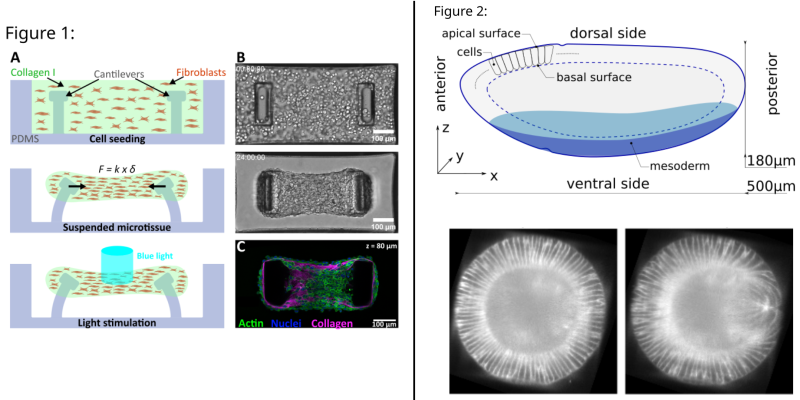
Figure 1: A. Schematic side-view of microtissue formation and stimulation in PDMS microwells. Force F is given by the deflection d and the spring constant k of the pillars B. Top-view brightfield images C. Top-view confocal image of a microtissue D. Mean Relative Force Increase upon a 500 ms light pulse (n = 8).
*******************************************************************************
Active viscoelasticity and shell mechanics in tissue morphogenesis

Antoine JALLON (PhD at MC2, Liphy)
Morphogenesis is the process by which living organisms acquire their shape. To study the mechanics of tissue folding, a ubiquitous morphogenetic event that allows 2D sheets of biological tissues to transition into 3D structures, we use the Drosophila Melanogaster (fruit fly) embryo as a model organism. During its early development, this nearly ellipsoidal cell monolayer folds and allows the internalisation of a subpopulation of cells, the mesoderm.
Here, we investigate the impact of viscoelasticity, as well as the mechanical role of the basal surface (see the figure) of the embryo, on this folding process. We first explain how a liquid-like rheology emerges both at the tissue and cell scale in developing tissues. We then present a method and a model that enables the description of active viscoelastic thin spherical shells. Finally, we show a related model investigating the role of basal-apical interaction on tissue folding.
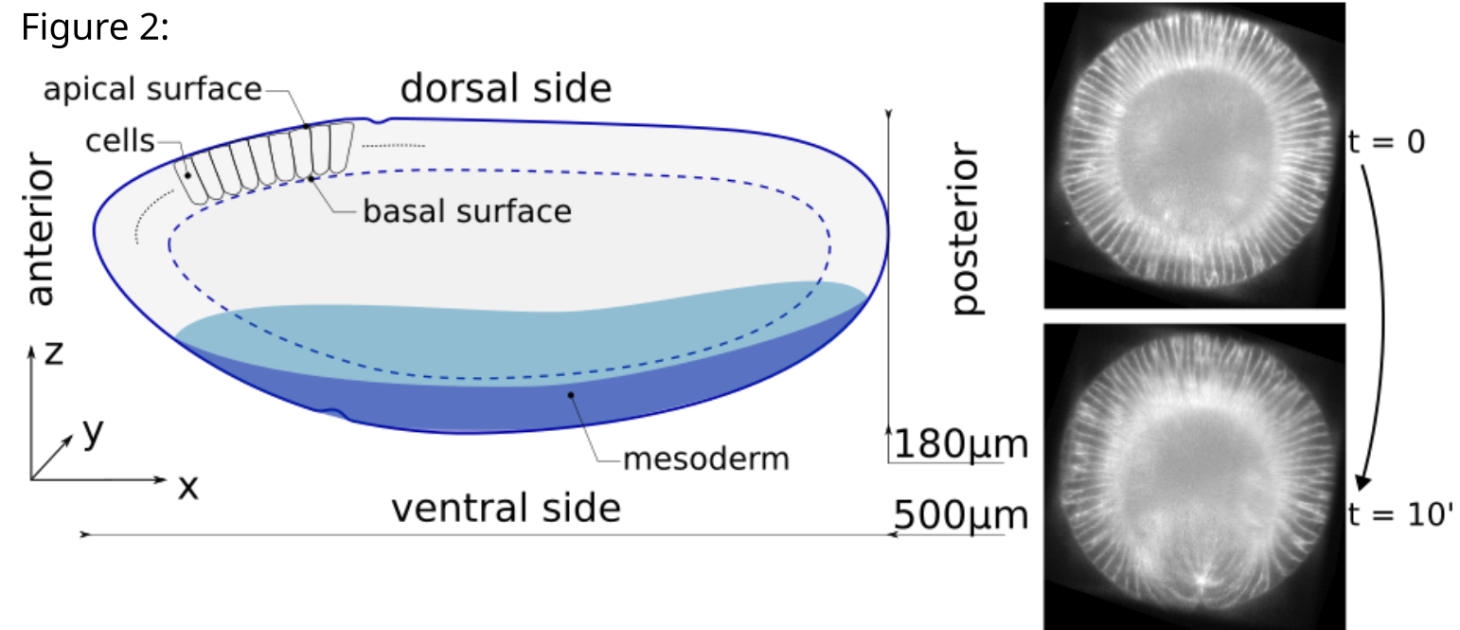
Figure 2 (left): Sketch of a drosophila embryo. Cells are represented in 2D only on the upper left but surround the whole embryo. The basal (resp. apical) surface is defined as the collective union of basal (resp. apical) domains of cells.
Figure 2 (right): Cross section of the embryo (along the zy plane) before and after the fold forms.
Date
11:00
Localisation
LIPhy, salle de conférence
- Imprimer
- Partager
- Partager sur Facebook
- Partager sur X
- Partager sur LinkedIn